
Evaluation of efficacy and safety of Liv.52 DS tablets in acute viral hepatitis:
A prospective, double-blind, randomized, placebo-controlled,
phase III clinical trial
Dr. Rajiv Baijal, M.D., D.N.B.1, Dr. Nikhil Patel, M.D.,2 and Dr. S. A. Kolhapure, M.D.3*
1Gastroenterologist, 2Research Associates, Department of Gastroenterology,
Jagjivanram Western Railway Hospital, Mumbai Central, Mumbai, India. 3Senior Medical Advisor, R&D Center, The Himalaya Drug Company, Bangalore, India
Liv.52 DS tablets in acute viral hepatitis
| Table 3: Improvement in mean symptom score of fatigue | |||||||
| Repeated Measures ANOVA Test | |||||||
| Parameter | Day 0 | 1stmonth | 2nd month | 3rd month | |||
| Mean | 2.52 | 1.52 | 1.00 | 0.00 | |||
| Std. Deviation | 0.51 | 0.51 | 0.00 | 0.00 | |||
| Std. Error | 0.10 | 0.10 | 0.00 | 0.00 | |||
| Lower 99% CI | 2.24 | 1.24 | 1.00 | 0.00 | |||
| Upper 99% CI | 2.81 | 1.81 | 1.00 | 0.00 | |||
| Significance | ***F=367, R 2 =0.9386, p <0.0001, Highly significant | ||||||
| Bonferroni's Multiple Comparison Test | |||||||
| Mean Diff. | t | p value | 99% CI of Diff. | ||||
| Day 0 vs 1 st month | 1 | 12.66 | p <0.001 | 0.754 to 1.245 | |||
| 1 st month vs 2 nd month | 0.52 | 6.58 | p <0.001 | 0.274 to 0.765 | |||
| 2 nd month vs 3 rd month | 0 | 0 | p >0.05 | -0.245 to 0.245 | |||
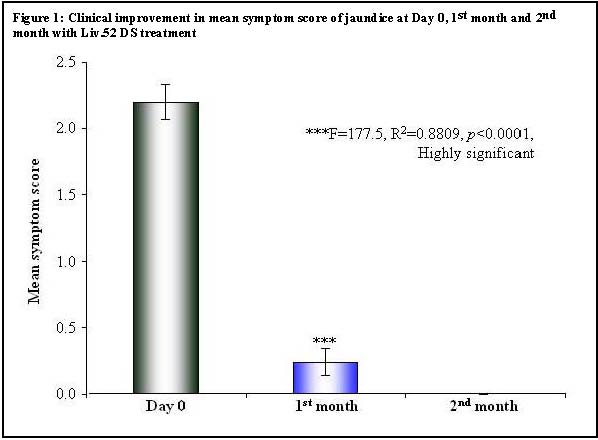
| Table 4: Clinical Improvement in mean symptom score of jaundice | ||||||
| Repeated Measures ANOVA Test | ||||||
| Parameter | Day 0 | 1 >st month | 2nd month | |||
| Mean | 2.20 | 0.24 | 0.00 | |||
| Std. Deviation | 0.65 | 0.52 | 0.00 | |||
| Std. Error | 0.13 | 0.10 | 0.00 | |||
| Lower 99% CI | 1.84 | -0.05 | 0.00 | |||
| Upper 99% CI | 2.56 | 0.53 | 0.00 | |||
| Significance | ***F=177.5, R 2 =0.8809, p <0.0001, Highly significant | |||||
| Bonferroni's Multiple Comparison Test | ||||||
| Mean Diff. | t | p value | 99% CI of Diff. | |||
| Day 0 vs 1 st month | 1.96 | 19.18 | p <0.001 | 1.643 to 2.277 | ||
| 1 st month vs 2 nd month | 1.67 | 12.38 | p <0.001 | 0.077 to 0.553 | ||
| 2 nd month vs 3 rd month | 0.00 | 0.00 | ||||
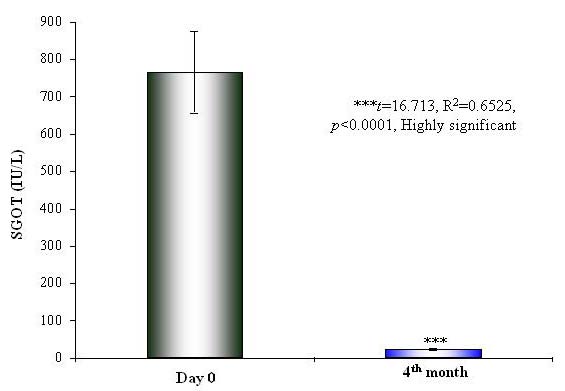
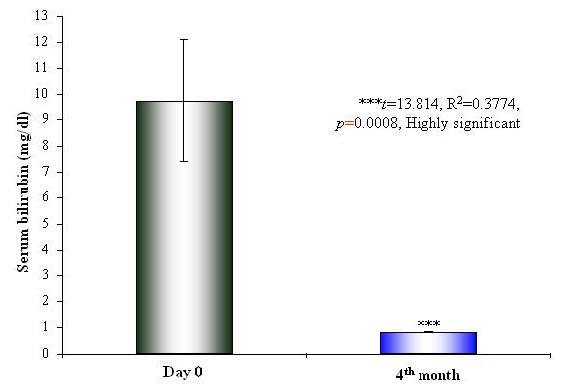
There was significant renormalization of the biochemical parameters of liver functions after 2 months in the Liv.52 DS group and after 3 months in the placebo group. There was a highly significant (p < 0.0001) reduction in the levels of SGOT (p < 0.0001) (Table 5 and Figure 2), SGPT (p < 0.0001) (Table 6 and Figure 3) and SB (p=0.0008) (Table 7 and Figure 4) in the Liv.52 DS group. There was highly significant improvement in the blood proteins, and the levels of SA (p < 0.0001) (Table 8), SG (p=0.0083) (Table 9) and TP (Table 10) were renormalized. The elevated levels of ESR (p=0.002) (Table 11 and Figure 5) and WBC (p=0.0412) (Table 12) were significantly reduced in the Liv.52 DS group, as compared to the placebo group, at the end of the study. There were no clinically significant changes in other biochemical parameters as Hb levels (Table 13) and PC (Table 14).
|
There were no clinically significant adverse effects, either observed or reported; during the entire study period, and the overall compliance to the drug treatment was found to be excellent. DISCUSSION Hepatitis A is an acute, but benign form of viral hepatitis caused by an RNA virus and the virus does not persist in the blood serum. Hepatitis A virus (HAV) is an enterovirus group of the picornaviridae family and HAV has a single molecule of RNA surrounded by a small (27 nm diameter) protein capsid. Hepatitis A is a food/waterborne disease, with feco-oral transmission. The incubation period is variable (from 15 to 25 days) and is inversely proportional to infective dose (presumably 10-100 virus particles). |
|||||||||||||||||||||||||||||||||||||||||||||
Figure 2: Improvement in SGOT levels at Day 0 and 4th month with Liv.52 DS treatment
Hepatitis A is usually a mild illness characterized by sudden onset of fever, malaise, nausea, anorexia and abdominal discomfort, followed in several days by jaundice. The dark urine (which precedes jaundice by 2/3 days) indicates the onset of the disease. The risk of transmission is generally low amongst household contacts, but outbreaks occur in nurseries and institutions with attack rates around 10%-15%. The duration of viral shedding is upto 14 days; with a sharp fall off after 5 days, but the period of infectiousness is about 8 to 17 days (the stool remains infectious upto 15 days). Asymptomatic infections are frequent. The serial interval between index and secondary cases is either shorter or equal to incubation period, indicating that transmission usually occurs before or around onset of jaundice.1
|
The HBV is a double-stranded DNA virus in the hepadnaviridae family. Hepatitis B virus is transmitted horizontally (by blood, blood products and sexual transmission) and vertically (from mother to infant in the perinatal period, which is a major mode of transmission in endemic regions). Hepatitis B virus causes acute and chronic hepatitis and the chances of becoming chronically infected depend upon age (about 90% of infected neonates and 50% of infected young children will become chronically infected and in contrast, only about 5% to 10% of immunocompetent adults infected with HBV develop chronic hepatitis B). Acute hepatitis B can range from subclinical disease to fulminant hepatic failure and about 90% of acutely infected adults recover without sequelae, while about 5% of acutely infected adults become chronically infected. Chronic infection with HBV can be either |
|||||||||||||||||||||||||||||||||||||||||||||
Virtually all individuals infected with HBV will have detectable serum HBsAg. In acute infection, HBsAg is detectable several weeks after infection and its appearance coincides with the onset of clinical symptoms. At around the same time, IgM antibodies against core antigen are detectable in serum and subsequently, IgG antibodies against core are produced. As acute infection resolves, IgG antibodies against core antigen persist and IgM antibodies and HBsAg become undetectable. Most people who have had acute infection that resolves continue to have IgG antibodies against core antigen for life. Acutely infected individuals who do not clear HBV continue to have serum HBsAg. In most cases, the chronic infection becomes "non-replicative" and the subjects lose serum HBeAg and develop antibodies against HBeAg. In some cases, "replicative" infection persists along with detectable serum HBeAg. In chronically infected individuals, infection can switch from "non-replicative" to "replicative" and vice-versa. One goal of treatment is to convert patients with chronic hepatitis B from a "replicative" (HBeAg positive) to "non-replicative" (HBeAg negative) state. Individuals with chronic hepatitis B are at increased risk for the development of hepatocellular carcinoma, and should be screened by serum alpha-fetoprotein and ultrasonography examination.

Copyrights © 2009 healthyliver.co.uk
